TOMSK STATE UNIVERSITY |
division of organic chemistry |
Structure and reactivity of organic compounds. Theory of organic compounds structure |
The main provisions of the theory of chemical structure
The structure of the carbon atom
Types of bonds in molecules of organic substances
Types of reactions in organic chemistry
Classification of organic compounds
Homologues and homology series
Isomerism of organic compounds
Electronic effects in organic molecules
Raw materials sources of organic compounds are oil and natural gas, bituminous and brown coal, oil shale, peat, agricultural and forestry products.
The criterion for dividing compounds into organic and inorganic is their elemental composition.
Organic compounds include chemicals that contain carbon, for example:
CH3-CN, CH3-CH2-OH, CS2, CH3COOH, CH3-NH2, CH3-NO2, CH3-COOC2H5.
Organic compounds differ from inorganic compounds in a number of characteristic features:
· Almost all organic substances burn or are easily destroyed when heated with oxidants, releasing CO2 (by this criterion, it is possible to establish the belonging of the investigated substance to organic compounds);
· In the molecules of organic compounds, carbon can be combined with almost any element of the periodic table;
· Organic molecules can contain a sequence of carbon atoms connected in chains (open or closed);
· The molecules of most organic compounds do not dissociate into sufficiently stable ions;
· The reactions of organic compounds proceed much more slowly and in most cases do not reach the end;
· Among organic compounds isomerism is widespread;
· Organic substances have lower temperatures of phase transitions (boiling and melting points).
There are much more organic compounds than inorganic.
The main provisions of the theory of chemical structure
1. Atoms in molecules are connected to each other in a certain sequence according to their valences. The sequence of interatomic bonds in a molecule is called its chemical structure and is reflected by one structural formula (structure formula).
2. The chemical structure can be established by chemical methods. (Currently, modern physical methods are also used).
3. The properties of substances depend on their chemical structure.
4. By the properties of a given substance, you can determine the structure of its molecule, and by the structure of the molecule, you can predict the properties.
5. Atoms and groups of atoms in a molecule have a mutual influence on each other.
Even from the moment when researchers learned to determine the elemental composition of compounds, it was noticed that often compounds with the same elemental composition have completely different chemical and physical properties. Revealing the reasons for this behavior stimulated the creation of a theory of the structure of organic compounds.
The theory of OC structure was the scientific foundation of organic chemistry and contributed to its rapid development. Based on the theory, an explanation for the phenomenon of isomerism was given, the existence of various isomers was predicted and some of them for the first time were obtained .
The structure of the carbon atom
It is obvious that all the reactions that organic molecules enter into are associated with the structure of the carbon atom of a particular molecule and the rearrangement of its outer valence orbitals during the transformation.
In an unexcited state, a carbon atom has 2 electrons in the s-orbital of the second sublevel (2s-orbitals), and also 2 electrons in two (out of a total of 3) p-orbitals of the 2-sublevel (2px and 2py orbitals):
2s |
2px |
2py |
2pz |
↑↓ |
↑ |
↑ |
As a result of receiving energy from the outside, a carbon atom passes into an excited state when one of the 2s electrons goes over to an energetically higher 2p orbital:
2s |
2px |
2py |
2pz |
↑ |
↑ |
↑ |
↑ |
Thus, in the outer orbitals, carbon has 4 electrons capable of forming bonds. According to the theory, the forms of s- and p-orbitals describe the probability of finding an electron relative to the nucleus of an atom. Non-hybridized s- and p-orbitals have the shape of a sphere and a uniform "dumbbell" and are located in space according to the scheme below:

When compounds are formed from atomic carbon (or in the composition of carbon compounds), there is a change in the shape and arrangement in space relative to the nucleus of the outer orbitals of carbon, called hybridization. Hybridization can be schematically represented as follows:

From four unhybridized atomic s- and p-orbitals having different shapes, as a result of sp3-hybridization (which means a change in one s- and three p-orbitals), four hybridized molecular orbitals, equivalent in energy and shape, are obtained, having the shape of a distorted dumbbell.
To ensure minimal steric hindrances and mutual repulsion, these four equivalent orbitals are located in space at equal distances from each other, directed to the vertices of the tetrahedron (the nucleus of the carbon atom is located in the center of the tetrahedron), and the spatial angles between the orbitals are about 109 ° 28 ':

In this state, four bonds as a result of overlapping orbitals can be formed without hindrance. In such hybridization, carbon is present (exclusively) in the composition of alkanes, cycloalkanes and alcohols.
Thus, for example, the ethane molecule looks like (yellow spheres show hydrogen atoms, more precisely, their s-orbitals):

The bond between carbon atoms is formed by overlapping hybridized orbitals. Such links are called s-links (sigma links). Molecule fragments can rotate around the s-bond.
Hybridization is a change in the shape and location in space relative to the nucleus of an atom of its external electronic orbitals, during the formation of bonds with other atoms. Another definition: hybridization - mixing of orbitals, as a result of which they align in shape and energy.
A carbon atom carrying a multiple bond (alkenes -C = C-, carbonyl compounds> C = O, carboxylic acids and their derivatives -COOH, -COOR, etc.) has a different hybridization (sp2), respectively, the form and the location in space of the outer orbitals:

In the state of sp2 hybridization at carbon, there are only 3 hybridized orbitals (obtained from one s and two p orbitals), which are located in one plane at an angle of 120 ° between them, and the fourth (unhybridized) p orbital is located perpendicular to this plane. A double bond is formed as a result of overlapping unhybridized orbitals between adjacent carbon atoms (or between carbon and oxygen), the figure shows an ethylene (ethene) molecule:

The bonds formed by overlapping unhybridized p-orbitals are called p-bonds. Thus, the multiple (double) bond in the ethene molecule is formed by one sigma and one pi-bond.
For obvious reasons, rotation of the fragments of the molecule around the p-bond is impossible at normal temperature (additional energy is required to break the overlapping p-orbitals), this determines the presence of spatial (geometric) isomers in alkenes, in the presence of some additional conditions, which will be discussed below.
In the figure, unhybridized p-orbitals are at a distance - artificially spaced for better perception, although in reality they "touch" each other, overlapping from above and below, but forming only one additional connection.
Carbon with a triple bond (in alkynes and nitriles) is in a state of sp-hybridization:

A pair of hybridized orbitals are arranged in a line, at an angle of 180 ° and oppositely directed. Two unhybridized p-orbitals, according to the principle of minimum repulsion and to minimize steric hindrances, are located perpendicular to this line and at an angle of 90 ° to each other. The triple bond in alkynes is formed by overlapping hybridized orbitals (one s-bond) and two unhybridized p-orbitals of adjacent carbon atoms (two p-bonds). So, for example, the model of the acetylene molecule (ethin) looks like:

As a result of the reactions, carbon is able to both change and maintain the state of its hybridization.
Types of bonds in molecules of organic substances
The predominant type of bond in the molecules of organic compounds is the covalent bond. A pair of bond electrons is divided approximately equally between the atoms, if you characterize the C-C or C-H bonds. This is due to the approximately equal electron affinity (electronegativity) of the C and H atoms.
In the case when carbon is bonded to a more electronegative atom (halogens, oxygen, nitrogen), the bond can be largely polarized, and partial positive (on carbon) and negative (on halogen, oxygen, nitrogen atoms) charges can form on the atoms. However, the degree of ionicity of such a bond is minimal.
Due to the non-polarity of the C-C and C-H bond, the predominant way of breaking it is homolytic, when a pair of electrons is divided equally between the atoms. This breaking of the bond creates uncharged but highly reactive particles with unpaired electrons called radicals. For alkanes, reactions with intermediate formation of radicals are very characteristic. Such transformations are initiated by the introduction from the outside of energy sufficient to break the bond (heating) or compounds that initiate the formation of radicals upon weak heating or ultraviolet irradiation (peroxides, halogens, azo compounds, chemical initiators that generate radicals as a result of a chemical reaction). In general, unstressed ring alkanes and cycloalkanes are chemically relatively inert.
In contrast, alkenes are much more reactive. The reason for this is the unsaturation (multiple bond) and the availability of loose electron density of overlapping p-orbitals of the p-bond for the action of electrophilic reagents (compounds with empty external orbitals or electron-deficient compounds). As a result, the multiple bonds disappear and electrophiles are added. Reactions proceed with the intermediate formation of positively charged intermediates (carbocations) or radicals.
Another group of reactions is associated with the polarization of the carbon-halogen, oxygen or nitrogen bond. These reactions have a more complex mechanism and depend on the structure of the substrate, reagent and reaction conditions (solvent, catalyst, etc.).
There are also more complex types of reactions (cycloaddition or the Diels – Alder reaction), the detailed mechanism of which has not yet been studied in details.
Types of organic chemistry reactions
Thus, it is possible to distinguish only a few types of reactions in which organic compounds enter:
1) substitution reactions, when one atom (or group of atoms) is replaced by another atom (or group of atoms). The carbon skeleton remains unchanged. Reactions proceed through a preliminary cleavage of a bond followed by the formation of a new one;
2) addition reactions. They are characteristic of compounds with unsaturation (multiple bonds), as a result of which the addition of other molecules (hydrogen, water, halogens, oxygen, hydrogen halides, etc.) is possible;
3) the reaction of elimination, when molecules (water, ammonia, halogens, hydrogen halides, hydrogen, CO, CO2, etc.) are split off from the molecule of an organic compound. Such reactions are often named according to the type of the cleaved molecule, respectively, dehydration, deamination, dehalogenation, dehydrohalogenation, dehydrogenation, decarbonylation, decarboxylation, etc .;
4) condensation reactions, when there is an enlargement of the carbon skeleton of the molecule;
5) cracking (or cleavage) reactions, as a result of which the carbon skeleton is split into smaller molecules;
6) reduxtion/oxidation reactions, accompanied by the removal(or addition) of hydrogen molecules (a special case of the elimination or addition reaction), or with the simultaneous introduction of oxygen molecules (transformation of alcohols into aldehydes and ketones and, further, into acids);
7) isomerization reactions (or rearrangement of the carbon skeleton or cycles);
8) polymerization reaction, as a result of which long unbranched polymer molecules are obtained from small molecules (monomers). In living nature, examples of the formation of branched polymer molecules are known, the structural units of which are organic molecules of monosaccharides (carbohydrates).
Classification of organic compounds
Despite the variety of organic compounds, their molecules are based on chains and rings formed from carbon atoms. Compounds that contain only carbon and hydrogen are called hydrocarbons. In this case, part of the carbon valencies is spent on the formation of bonds with neighboring carbon atoms, and free valencies bind carbon with hydrogen, oxygen, nitrogen, sulfur and, much less often, with other atoms of the periodic system. Very often, such a "skeleton" of carbon atoms is preserved as a result of chemical transformations undergone by a molecule of an organic compound, which greatly facilitates the prediction of the composition of products. Often, reactions are limited to the replacement of one or more hydrogen atoms with another element or group of atoms (otherwise called a group or functional group), as a result of which an organic compound of a different class is obtained. Depending on the grouping that has replaced one of the hydrogen atoms in the molecule of the organic compound as a result of the reaction, classes of organic compounds are distinguished.
Often, as a result of the reaction, one functional group is replaced by another, while maintaining the carbon skeleton. However, numerous reactions are also known, accompanied by a change in the carbon skeleton of the molecule.
Some functional groups of organic compounds
Group |
Name |
Class |
General structure |
Sample |
-Cl, -F, -Br, -I (-Õ) |
Halogen |
Halides |
R-X |
Bromobenzene |
Etenyl chloride (vinyl chloride) |
||||
-ÎÍ |
Hydroxyl (oxy-, hydroxy-) |
Alcohols, Phenols |
R-OH |
Phenol |
Methanol |
||||
>Ñ=Î |
Carbonyl (oxo) |
Aldehydes, ketones |
|
Propanone (acetone)
Ethanal (acetaldehyde) |
-ÑÎÎÍ |
Carboxyl (carboxy) |
Carboxylic acids |
|
Ethanic acid (acetic acid) |
-NO2 |
Nitro |
Nitro compounds |
|
Nitromethane |
-NH2 |
Amino |
Amines |
|
Aminomethane (methylamine) |
-CN |
Cyano |
Nitriles |
|
Ethanolnitrile (acetonitrile) |
Homologues and homology series
Homologues are organic compounds (of the same class, see above), differing by one or more methylene groups (-CH2- units). Homologues for alkanes are, for example, methane, ethane, propane, butane, etc., in which the number of carbon atoms changes by one (or by the same number of methylene units).
Homologues of aromatic compounds are benzene, toluene, xylenes, mesitylene, ethylbenzene, and other alkyl-substituted benzenes. These compounds also differ in gross formula by one or more methylene units (-CH2-). Accordingly, homologues are methanol, propanol and ethanol, acetone and methyl ethyl ketone, acetic and propionic acids, etc.
Isomerism of organic compounds
The structure formula (structural formula) describes the order in which atoms are joined in a molecule, i.e. its chemical structure. Chemical bonds in the structural formula are represented by dashes. The bond between hydrogen and other atoms is usually not indicated (such formulas are called abbreviated structural).
Structural formulas differ from molecular (gross) formulas, which show only which elements and in what ratio are included in the composition of a substance (i.e., the qualitative and quantitative elemental composition), but do not reflect the order of binding of atoms. For example, n-butane and isobutane have the same molecular formula C4H10, but different bond sequences.
Thus, the difference in substances is due not only to different qualitative and quantitative elemental composition, but also to different chemical structures, which can be reflected only by structural formulas. Even before the creation of the theory of structure, substances of the same elemental composition, but with different properties, were known. Such substances were called isomers, and this phenomenon itself was called isomerism. Isomerism, as shown by A.M. Butlerov, there is a difference in the structure of molecules consisting of the same set of atoms. Thus, isomerism is the phenomenon of the existence of compounds that have the same qualitative and quantitative composition, but different structures and, therefore, different properties.
For example, if a molecule contains 4 carbon atoms and 10 hydrogen atoms, two isomeric compounds may exist:

Depending on the nature of the differences in the structure of isomers, structural and spatial isomerism are distinguished.
Structural isomerism
Structural isomers are compounds of the same qualitative and quantitative composition, differing in the order of bonding of atoms, that is, in their chemical structure.
For example, the composition C4H8 corresponds to 5 structural isomers:

Among organic compounds, the existence of a colossal amount of only structural isomers is theoretically possible. So, among alkanes containing only carbon and hydrogen atoms, the number of possible isomers increases exponentially with an increase in the number of carbon atoms. If for a compound of composition C4H10 only two isomers can exist, then for pentanes C5H12 the number of such isomers increases to three, C6H14 has 5 isomers, C7H16 has 9 isomers, C8H18 has 18 isomers, C9H20 has 35 isomers, and for pentacosane C25H52 it is theoretically possible to exist neither more nor less - 36 797 588 isomers.
In the above example, the following isomers can be distinguished:
- the position of the double bond (butene-1 and butene-2);
- carbon skeleton (butenes-1 and -2 and isobutylene);
- cycle sizes (cyclobutane and methylcyclopropane);
- interclass isomers (alkenes and cycloalkanes).
Interclass isomers are, for example, ethanol and dimethyl ether, which have the same gross formula C2H6O, but completely different structures and belong to different classes. They differ not only in chemical properties (the more inert dimethyl ether does not react with metallic sodium, unlike ethanol), but also in physical properties. Ethanol is liquid at normal temperature, while dimethyl ether is a gas.
Cyclic and acyclic organic compounds
It can be noted that among the structural isomers of organic compounds, there may be molecules containing in their composition rings built from carbon atoms of different numbers (and often more than one such cycle in the composition of the molecule). On this basis, a distinction is made between alicyclic compounds (containing rings, or simply cyclic compounds) and acyclic compounds (not containing rings, but built exclusively from chains of carbon atoms, often branched).
Carbocyclic compounds contain only carbon atoms in the ring. They are divided into two groups that differ significantly in their chemical properties: aliphatic cyclic (abbreviated alicyclic) and aromatic compounds.
Heterocyclic compounds contain in the cycle, in addition to carbon atoms, one or more atoms of other elements - heteroatoms (from the Greek heteros - other, different) - oxygen, nitrogen, sulfur, etc.
Spatial isomerism
Spatial isomers (geometric isomers, stereoisomers) with the same composition and the same chemical structure differ in the spatial arrangement of atoms in the molecule.
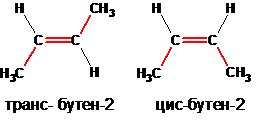
The spatial isomers are optical (mirror) and cis-trans isomers. In the example shown below, butene-2, which exists in nature in the form of cis- and trans-butenes-2, can have spatial isomers:
Spatial isomerism appears, in particular, when carbon has four different substituents:

If you swap any two of them, you get another spatial isomer of the same composition. The physicochemical properties of these isomers differ significantly. Compounds of this type are distinguished by the ability to rotate the plane of the polarized light transmitted through the solution of such compounds by a certain amount. In this case, one isomer rotates the plane of polarized light in one direction, and its isomer - in the opposite direction. Due to these optical effects, this type of isomerism is called optical isomerism.
More details on optical isomerism can be found in the section on oxygen-containing and nitrogen-containing organic compounds.
Optical isomerism is a special case of spatial isomerism. Optical isomers are called molecules differing in the spatial arrangement of groups and atoms, having the same composition and the same bond order of atoms. Solutions of such compounds are able to rotate the plane of the polarized light transmitted through them by a certain angle.
Nomenclature of organic compounds
Due to the presence of a huge number of organic compounds, the system of their designation (naming) is of great importance in such a way that the name can easily establish its structure (chemical structure), and, accordingly, all chemical and physical properties. Thus, the name should reflect the chemical structure of the organic compound as accurately as possible, including the ability to identify structural and geometric isomers. To date, there are three types of nomenclature of organic compounds:
1.trivial;
2. rational;
3. systematic (or substitutional, or IUPAC nomenclature).
The presence of trivial names is associated with history. Previously, researchers often gave names to compounds by the source of their isolation or by some organoleptic, physicochemical properties. Trivial names are in circulation sometimes with the same rights (if not more often) than systematic names. For example, the name acetic acid, formic acid, lactose, urea and many other names are still used.
Rational nomenclature
This type of nomenclature has become widespread as a result of the fact that some of the compounds can be named as a kind of parent compound, from which they differ in substituents. An example is neopentane ("new pentane"), a hydrocarbon of the C5H12 alkane class. The name "neopentane" is considered trivial, and says absolutely nothing about its structure. According to the nomenclature of the second type, this hydrocarbon can be called tetramethylmethane. The name tetramethylmethane is already much more informative in terms of information on the structure of the molecule. One can imagine a methane molecule in which all four hydrogen atoms have been replaced by methyl groups.
The systematic name of neopentane is the name 2,2-dimethylpropane, compiled according to the rules developed by the International Union of Pure and Applied Chemistry (IUPAC). The structural formula of neopentane is shown below:

We will do a detailed consideration of the rules for naming organic compounds later, when considering individual classes of organic compounds, since each case has its own characteristics.
Electronic effects in organic molecules
The replacement of hydrogen atoms in alkane molecules by any heteroatom (halogen, nitrogen, sulfur, oxygen, etc.) or group causes a redistribution of the electron density. The nature of this phenomenon is different. It depends on the properties of the heteroatom (its electronegativity) and on the type of bonds along which this influence propagates.
Inductive effect
If the influence of a substituent is transmitted with the participation of s-bonds, then there is a gradual change in the electronic state of the bonds. This polarization is called the inductive effect (I) and is depicted by an arrow in the direction of the electron density shift. The electron density always shifts towards a MORE ELECTRIC NEGATIVE atom or group of atoms:
ÑÍ3-ÑÍ2-->Cl,
HO <-- ÑÍ2-ÑÍ2 --> Cl,
ÑÍ3-ÑÍ2 --> COOH,
ÑÍ3-ÑÍ2 --> NO2 è ò.ä.
The inductive effect is due to the tendency of an atom or a group of atoms to supply or pull off the electron density, and therefore it can be positive or negative. Elements more electronegative than carbon exhibit a negative inductive effect, i.e. halogens, oxygen, nitrogen and others, as well as groups with a positive charge on an element bound to carbon. The negative inductive effect decreases from right to left in the period and from top to bottom in the group of the periodic system:
F > O > N,
F > Cl > Br > J.
In the case of fully charged substituents, the negative inductive effect increases with an increase in the electronegativity of the atom bonded to carbon:
>O+- >> N+< .
In the case of complex substituents, the negative inductive effect is determined by the nature of the atoms that make up the substituent. In addition, the inductive effect depends on the nature of the hybridization of the atoms. Thus, the electronegativity of carbon atoms depends on the hybridization of electron orbitals and changes in the following direction:
sp3 < sp2 < sp.
Elements less electronegative than carbon have a positive inductive effect; groups with a full negative charge; alkyl groups. + I-effect decreases in the series:
(ÑÍ3)3Ñ- > (CH3)2CH- > CH3-CH2- > CH3- > H-.
The inductive effect of the substituent rapidly dies out as the chain length increases.
Table
Summary table of substituents and their electronic effects
A substituent or group of atoms (X=halides) |
Effect |
-ÑÍ3 > CH3-CH2- > (CH3)2CH- >> CH2X |
+I, +M |
(CH3)3C- |
+I, M = 0 |
An atom attached to a p-system has a lone pair of electrons: (X=halides), -O-, -OH, -OR, -NH2, -NHR, -NR2, -SH, -SR, |
–I, +M |
the atom attached to the p-system, in turn, is associated with a more electronegative atom: -N=O, -NO2, -SO3H, -COOH, -CO-H, -CO-R, -CO-OR, -CN, -ÑÍÕ2, -CX3, -C=N=S |
–I, –M |
More electronegative carbon (compared to sp3): -ÑÍ=ÑÍ-, -Ñ=ÑÍ (ethynyle), -Ñ6Í4- (phenylene) (but easily transfer the M-effect in any direction) |
–I, M = 0 |
An atom with no p-orbitals, but with a full positive charge -N+H3, -N+R3 , (-S+R2 ,-O+H2 ), |
–I, M = 0 |
Mesomeric effect
The presence of a substituent with a free pair of electrons or a vacant p-orbital attached to a system containing p-electrons leads to the possibility of mixing the p-orbitals of the substituent (occupied or vacant) with p-orbitals and a redistribution of the electron density in the compounds. This effect is called mesomeric.
The shift in the electron density is usually insignificant and the bond lengths practically do not change. An insignificant shift of the electron density is judged by the dipole moments, which are small even in the case of large conjugation effects on the extreme atoms of the conjugated system.
The mesomeric effect is depicted by a curved arrow directed towards the displacement of the electron density. The electron density is always shifted towards a more electronegative atom located at the edge of the structure and connected to the rest of the structure by a multiple bond:

Depending on the direction of the displacement of the electron cloud, the mesomeric effect can be positive (+ M), an atom or when a group of atoms transfer electrons to the pi-system:

and negative (-M), when the group of atoms pulls electrons from the pi system:

The positive mesomeric effect (+ M) decreases with an increase in the electronegativity of the atom carrying the lone pair of electrons, due to a decrease in the tendency to give it away, as well as with an increase in the volume of the atom. The positive mesomeric effect of halogens changes in the following direction:
F> Cl> Br> J (+ M-effect).
A positive mesomeric effect is possessed by groups with lone pairs of electrons on an atom attached to the conjugated pi system:
-NH2 (NHR, NR2)> OH (OR)> X (halogen) (+ M-effect).
The positive mesomeric effect decreases when the atom is bound to an electron acceptor group:
-NH2> -NH-CO-CH3.
The negative mesomeric effect increases with an increase in the electronegativity of the atom and reaches its maximum values ??if the acceptor atom carries a charge:
> C = O + H >>> C = O.
A decrease in the negative mesomeric effect is observed if the acceptor group is conjugated with the donor group:
-CO-O- << -CO-NH2 <-CO-OR <-CO-H (R) << -CO- CO- <-CO-X (halogen) (–M-effect).
Hyperconjugation
A positive mesomeric effect occurs when hydrogen is replaced at a multiple bond by an alkyl group. This effect is directed towards the multiple bond and is called hyperconjugation (overconjugation):

The effect resembles a positive mesomeric effect, since it donates electrons to the conjugated p-system:

Overconjugation decreases in the sequence:
CH3> CH3-CH2> (CH3) 2CH> (CH3) 3C.
For the manifestation of the hyperconjugation effect, the presence of at least one hydrogen atom with a carbon atom adjacent to the p-system is necessary. The tert-butyl group does not exhibit this effect, and therefore its mesomeric effect is zero.














