Chapter 2. Cycloalkanes (alicyclic
hydrocarbons)
In the composition of
cyclic alkanes, carbon is also in the state of sp3 hybridization, however, the
degree of unsaturation is higher there. The general formula of hydrocarbons in
a series of cycloalkanes is СnH2n. (Not to be confused with alkenes, which also
have the same gross formula).
2.2.1. Classification and nomenclature
Cycloalkanes differ in
ring size as well as in the number of rings in a molecule.
Among polycycloalkanes, there are compounds:
- with detached rings
(A);
- with rings connected
by a simple bond (B);
- rings having one
common carbon atom (C);
- rings having two or
more common carbon atoms (D):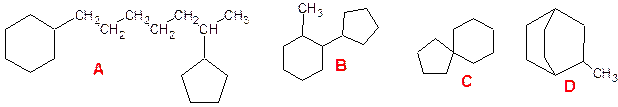
A is
1-cyclohexyl-6-cyclopropylheptane;
B-
1-methyl-2-cyclopentylcyclohexane;
C - Spiro [4.5] decane;
D- 2-methylbicyclo
[2.2.2] octane
When naming monocyclic compounds, the carbon atoms of the ring are
numbered so that the sum of the numbers is minimal, after which the available
substituents are listed and the name of the hydrocarbon corresponding to the
number of carbon atoms in the ring is added with the prefix cyclo-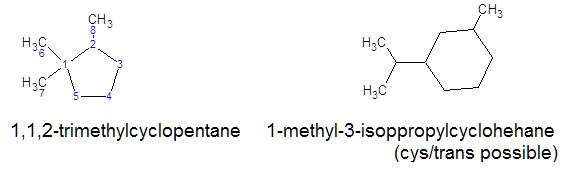 Compounds containing more than one cycle have
a more complex naming system.
Compounds containing more than one cycle have
a more complex naming system.
So, if the rings are isolated (A), the name is formed from an open-chain
hydrocarbon, and the rings act as substituents.
If the cycles are connected by a simple bond, one of them is called
radical (B).
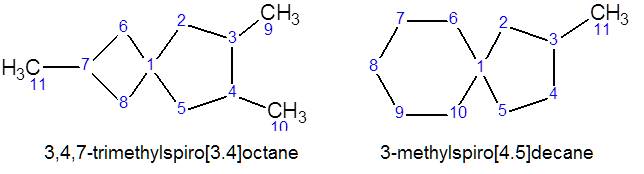
If the cycles have one common carbon atom (variant C), such molecules
are called spirains, in square brackets indicate the
number of carbon atoms in one and the other cycle, not counting the common
atom. The brackets are followed by the name according to the total number of
carbon atoms in the cycles, as if it were an open chain:
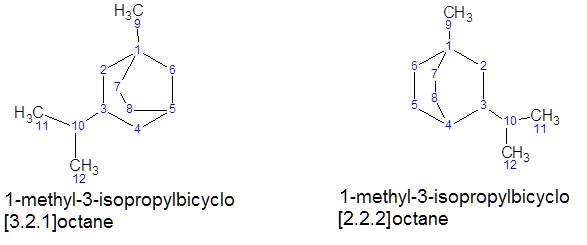
Polycyclic hydrocarbons with rings sharing two or more carbon atoms also
have carbon atoms called bridge heads. Numbering is carried out from the head
of the bridge along the path of the largest cycle, then the smaller cycle. The
substituents are listed with the numbers of the atoms of the rings to which
they are attached, the word bicyclo (for bicyclic
hydrocarbons) or tricyclo (for tricyclic) is added.
The numbers of carbon atoms in the cycle bridges are listed in square brackets,
separated by a dot, after which the name of the hydrocarbon is added
corresponding to the number of carbon atoms in the cycles (in total).
2.2.2. Isomerism of cycloalkanes
Cycloalkanes with the same number of carbon atoms in a molecule can have
not only structural isomers (by ring size and arrangement of substituents), but
also spatial isomers if the ring has two or more substituents.
Isomers differ in the arrangement of substituents relative to the plane
of the ring.
The figure shows the projection model of 1,2-dimethylcyclohexane. The
cyclohexane ring is in the chair conformation. (Red and blue in the figure show
the C-C bonds of the ring and substituents. Black - bonds with hydrogen atoms.
Hydrogen atoms are not shown).
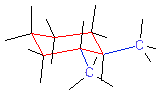
It can be seen that, depending on the position of the methyl substituents
relative to the ring, the existence of two isomers is possible: when both
substituents are located on one side of the ring, and when they are located on
opposite sides. The figure shows the trans isomer of 1,2-dimethylcyclohexane.
2.2.3. Cyclanes conformations
Due to the fact that the normal bond angles of the sp3-hybridized carbon
are 109 degrees with a small degree, only the cyclopropane ring can be flat,
where the angular stresses are very pronounced. Other cycles are far from the
plane state due to the fact that they adapt to the minimum angular stresses of
the bonds. So, for example, cyclohexane can be in two conformational states -
chair and bath:
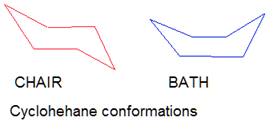
These states are capable of transforming into each other at ordinary
temperatures, and cannot be individually separable, therefore it makes no sense
to talk about the presence of isomers here. The state of the chair is
energetically more favorable, especially if there are substituents in positions
1 and 4.
2.2.4. Chemical properties of
cycloalkanes
The chemical properties of cycloalkanes depend on the size of the cycle
(up to a certain size). Small cycles, due to strong angular stress, have
increased internal energy and tend to react with ring opening. As the angular
stresses decrease (from cyclopropane to cyclobutane),
this reactivity drops sharply, and compounds such as cyclopentane
or cyclohexane strongly resemble alkanes in chemical ability.
Thus, compounds with the number of carbon atoms in the cycle of 7 or
more do not practically differ from cyclohexane either in specific heat of
hydrogenation or in other characteristics.
They are not inclined to enter into reactions accompanied by ring
opening; they are also characterized by substitution reactions proceeding by a
radical mechanism.
So, for cyclopropane
(and to a lesser extent for cyclobutane) the
following reactions are characteristic:
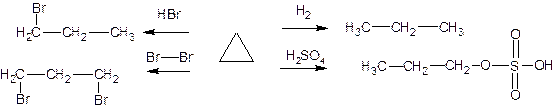
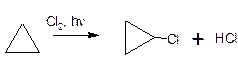
Under the action of chlorine, along with the rupture of the cycle, the
reaction of hydrogen substitution also takes place:
The presence of electron-withdrawing substituents (-CN, -Cl, -NO2,
-COOH, etc.) in the ring makes it difficult to open the rings, and the presence
of electron donor (alkyl groups) facilitates the opening of the rings.
2.2.5. Methods for the synthesis
of cycloalkanes
The effect of sodium metal on 1,3-dihalide derivatives of alkanes
(intramolecular Wurtz reaction):
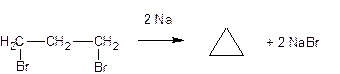
The same transformation proceeds even better with the action of zinc
dust in an alcoholic solution.
An important way to obtain three-membered rings is also the addition of carbenes to unsaturated compounds:
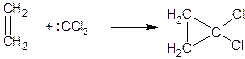
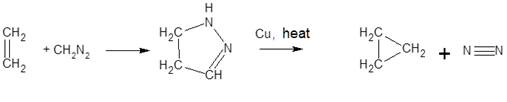
If diazo compounds are used for this purpose, heterocycles are first
formed, which, when heated in the presence of copper, release nitrogen and form
cyclopropane derivatives:
The processes of dehydrocyclization of normal
and branched alkanes lead to the production of 5- and 6-membered cycloalkanes:
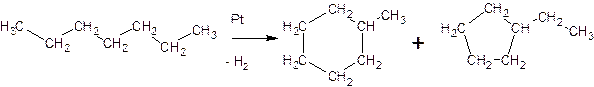
The production of rings with a large number of carbon atoms turned out
to be an unexpectedly trivial task. In any case, these methods cannot be
obtained. They were obtained much later in various indirect ways.